Transcription Factor Inhibitors: From “Undruggable” to Drug Discovery Reality
Why transcription factors were once “hard to drug” – and what changed
Transcription factors (TFs) regulate gene programs and usually lack deep pockets; they use broad protein-protein and protein-DNA interfaces and often contain intrinsically disordered regions. Structural biology, chemical biology, and new modalities have shifted the odds: we now have approved drugs that modulate TF output directly or indirectly, credible clinical signals for TF-directed agents, and maturing toolkits to discover and optimize TF binders (Bushweller 2019, Lambert 2018).
Structural & computational enablement
Cryo-EM, X-ray, NMR/HDX-MS, AlphaFold2-Multimer, and MD simulations reveal cryptic or allosteric pockets and hot spots on TF complexes (e.g. KIX, BTB, PAS, and nuclear receptors). These methods now routinely guide screen design and hit optimization, including for flat PPI surfaces and disordered regions (Bushweller 2019, Su 2021).
What counts as a “Transcription Factor Inhibitor”?
A transcription factor inhibitor can (1) block TF:DNA binding, (2) disrupt TF:cofactor PPI, (3) block TF:TF dimerization, (4) intercalate with DNA to block TF binding, or (5) inhibit subcellular shuttling of TF E(Figure 1A). Alternatively, TFs can be degraded through the proteasome degradation system either using PROTACs (Figure 1B) or molecular glue degraders. Most importantly, degradation already has human proof-of-concept: the IMiDs lenalidomide/pomalidomide recruit CRBN to gdegrade IKZF1/IKZF3 – bona fide TFs – hereby reprogramming myeloma cells. These agents prove that pharmacologic TF degradation is clinically feasible (Krönke 2013).
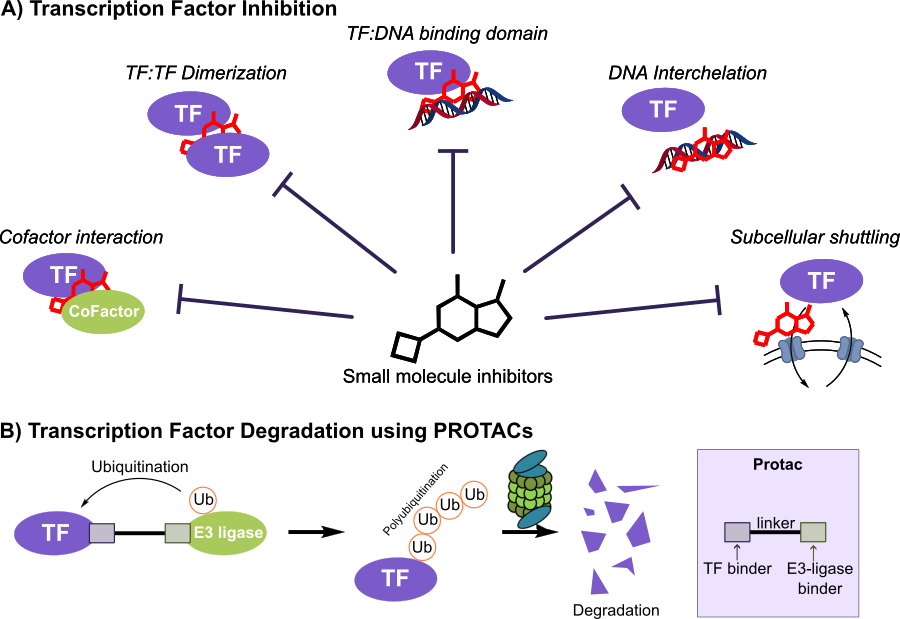
Figure 1: Different ways for small molecule transcription factor inhibition. A) Traditional ways for small molecule transcription factor inhibition and B) Transcription factor inhibition by PROTACs
Direct clinical and translational examples. HIF-2α (a TF dimer subunit) has an approved small molecule (belzutifan) for VHL-disease RCC (Jonasch 2021), and first-in-human TEAD palmitoylation inhibitors show activity in NF2-mutant tumors (Yap, 2023). A MYC-dominant negative mini-protein (OMO-103) delivered target engagement and early clinical signals (Garralda 2024). PROTACs that degrade STAT3 demonstrate potent clinical activity (Zhou 2019).
Modalities & case studies (small molecules to macrocycles)
- Orthosteric/allosteric small molecules. HIF-2α (belzutifan, approved), TEAD palmitoylation inhibitors (e.g., VT3989, Ph 1), and PPI modulators like BCL6 (79-6; FX1) or CREB (KG-501; 666-15) illustrate tractable TF surfaces and cofactors (Jonasch 2021, Yap 2023, Cerchietti 2011, Cardenas 2016, Best 2004).
- Targeted protein degradation. Besides CRBN-recruiting IMiDs (IKZF1/3), STAT3 PROTACs (e.g., SD-36) exemplify degrader strategies against TFs and co-regulators (Krönke 2013).
- Beyond small molecules. Constrained peptides, mini-proteins, macrocycles, and peptidomimetics expand reach to large PPIs. Omomyc (OMO-103), a myc dominant-negative mini-protein, achieved clinical target engagement and disease stabilization in a subset of patients (Gerralda 2024).
- Indirect TF pathway control. BET bromodomain inhibitors collapse super-enhancer programs (e.g. myc), CDK7/9 inhibitors throttle transcriptional pause-release/elongation, and CBP/p300 HAT inhibitors down-tune coactivator acetylation—all of which modulate TF-driven outputs. The KEAP1–NRF2 PPI inhibitors (e.g., KI-696) illustrate TF activation via PPI blockade, useful for oxidative-stress and inflammatory contexts.
How to find a Transcription Factor Inhibitor: assays that work
Biochemical assays for transcription factor screening
For TF:DNA disruption or TF:PPI modulation, choose a primary assay with an orthogonal biophysics confirm:
- Fluorescence polarization/anisotropy (FP/FA) using labelled DNA (or peptide cofactor) is robust and HTS-friendly; it tolerates inner-filter effects better than many readouts. Confirm hits by SPR/BLI/ITC (Hall 2016).
- AlphaScreen/AlphaLISA proximity assays scale to UH-TS. For example, a miniaturized AlphaScreen found inhibitors of the HMGA2:DNA interaction (Su 2020).
- Reporter assays (luciferase/SEAP) encode pathway-level TF activity; paired with CRISPRi/a or degrader controls, they triage direct vs indirect mechanisms. (Tao 2023)
Assay interferences & counterscreens (critical “hygiene”).
Guard against DNA intercalators/groove binders (run ethidium displacement, calf-thymus DNA counterscreens), colloidal aggregators (add low % detergent, DLS), and redox/fluorescence artifacts (redox scavengers; absorbance/fluorescence scans). Validate with SPR/BLI/ITC and enforce tag-switched controls. Close the loop with in-cell target engagement before chemistry scale-up (Hall 2016).
Cell-based target engagement (label-free or energy-transfer)
NanoBRET/NanoBiT target-engagement assays quantify compound binding in living cells; CETSA-MS profiles proteome-wide thermal shifts to reveal on- and off-targets and PD markers. These are particularly valuable for disordered or PPI-rich TFs where classical occupancy assays struggle.
DNA-encoded library (DEL) transcription factor inhibitor screening
DEL selections enable billions of compounds to be tested for TF binding at modest cost. Best practices for TFs: include polyanionic competitors (poly(dI–dC), heparin) and high-salt washes to suppress nonspecific capture by the DNA barcode; run tag-flipped negative selections and DNA-only baits; stabilize the biologically relevant TF complex (e.g., TEAD with palmitate, TF+cofactor). Prioritize hits with count-aware statistics (Poisson/Bayesian), then re-synthesize off-DNA for SPR/BLI and cell-based assays (Gironda-Martínez 2021).
Modern DEL analytics (including uncertainty-aware and 3D-aware ML) help denoise count data and surface chemotypes likely to validate after off-DNA synthesis.
Where DEL fits:
DELs complement medium-throughput transcription factor screening funnels (FP/Alpha/SPR) and can seed degrader ligand discovery (e.g., KEAP1-binding warheads). For practical context on platform variants, see vendor technical notes (e.g., Vipergen’s overview).
Translational realities: resistance, biomarkers, combinations
Disease areas beyond oncology
Most important TF drug targets
| Target/Complex | Modality | Representative agent(s) | Clinical Stage | One-line rationale |
|---|---|---|---|---|
| HIF-2α (EPAS1:ARNT) | Allosteric small molecule | Belzutifan (MK-6482) | Approved | Directly inhibits HIF-2α dimer function. Strong precedent for TF targeting. (Jonasch 2021). |
| TEAD (YAP/TAZ–TEAD) | Palmitoylation inhibitors | VT3989 | Phase 1 | Blocks YAP/TAZ transcriptional output. Activity in NF2-mutant tumors (Yap 2023). |
| MYC/MAX | Mini-protein/peptidomimetic | OMO-103 (Omomyc) | Phase 1 | First clinical-stage direct MYC inhibitor with target engagement (Garralda 2023). |
| p53/MDM2 | PPI antagonist | Milademetan | Phase 3 completed | Reactivates p53. Defined biomarker population (MDM2-amp). (Gounder 2023). |
| IKZF1/IKZF3 | Molecular glues (CRBN) | Lenalidomide, Pomalidomide | Approved | Clinically validated TF degradation drives myeloma efficacy (Krönke 2013). |
| STAT3 | PROTAC degrader | SD-36 | Preclinical | Potent degradation of STAT3 with strong in vivo activity. (Zhou 2019). |
| BCL6 (BTB corepressor hub) | PPI disruptor | 79-6, FX1 | Preclinical | Disrupts BTB:corepressor binding. Regression in DLBCL models. (Cerchietti 2010). |
| CREB:CBP/p300 (KIX) | PPI antagonist | KG-501, 666-15 | Preclinical | Blocks coactivator recruitment. Robust pathway inhibition in vivo. (Best 2004). |
| NF-κB | Indirect pathway modulators | Multiple | Mixed | Central inflammatory/oncogenic TF. Druggable via upstream nodes. (Verzella 2022). |
| ER (ESR1) | Orthosteric antagonists, SERDs | Tamoxifen class, etc. | Approved | Canonical TF target with decades of clinical validation. (Tremont 2017). |
| AR (NR3C4) | Antagonists | Enzalutamide | Approved | Improves survival in mHSPC. Classic nuclear receptor TF. (Davis 2019). |
| NRF2 (NFE2L2) | KEAP1–NRF2 PPI inhibitors (activators) | KI-696 (tool) | Preclinical | Keap1 PPI inhibitors elevate cytoprotective NRF2 programs. (Dinkova-Kostova 2023). |
| SMAD2/3/4 | Interface modulators. Degraders (emerging) | Various | Preclinical | Central to TGF-β signaling in fibrosis and cancer. (Bushweller 2019). |
| AP-1 (FOS/JUN) | PPI/disruption. Degrader concepts | Emerging | Preclinical | Oncogenic bZIP factors. Rich interface biology. (Bushweller 2019). |
| ETS family (ERG/ETV1/ETV6) | DNA-binding/PPI strategies | Emerging | Preclinical | Fusion-driven oncogenes. Tractable in principle via PPIs/DNA mimicry. (Bushweller 2019). |
Practical blueprint for transcription factor inhibitor screening
- Start biochemical, finish biophysical. Use FP/FA or AlphaScreen for throughput, then SPR/BLI/ITC to confirm direct binding and establish mechanism (DNA vs cofactor vs allosteric) (Hall 2016, Su 2020).
- Counterscreens early. DNA intercalation/groove-binding, colloidal aggregation, and redox fluorescence artifacts account for many false positives; bake in detergent, polyanions, and orthogonal readouts (Hall 2016).
- Go cellular quickly. Move to NanoBRET target engagement and CETSA-MS to verify in-cell binding and explore selectivity (Robers 2015, Savitski 2014).
- Use DEL for breadth, ML for triage. DEL campaigns with TF-specific safeguards (above) plus uncertainty-aware analytics markedly improve triage to off-DNA and cell follow-up (Gironda-Martínez 2021, Lim 2022).
- Lean on structure. Where possible, co-crystallize or use cryo-EM/NMR/HDX-MS to map pockets and hot spots, then iterate chemistry against those constraints (Bushweller 2019).
Final takeaway
- Undruggable no more: There are approved and clinical stage precedents for TF modulation.
- Effective transcription factor inhibitor screening blends robust biochemical assays, orthogonal biophysics, and in-cell engagement, with DELs and ML providing breadth and precision.
- Expect combinations and biomarker-driven development to be central as programs move from bench to bedside.
FAQ
Begin with a biochemical primary—e.g., fluorescence polarization for TF:DNA or AlphaScreen for TF:PPI—because they scale and are mechanism-specific. Immediately add counterscreens for DNA intercalation and colloidal aggregation, then confirm direct binding with SPR/BLI/ITC. Move quickly to cellular target engagement (NanoBRET or CETSA-MS) to avoid chasing artifacts. For breadth, run a DNA-encoded library (DEL) selection with TF-specific safeguards, then re-synthesize off-DNA for orthogonal validation.
References
- Bacon, C. W. and D’Orso, I., CDK9: a signaling hub for transcriptional control, Transcription, 10 (2), 57-75. https://doi.org/10.1080/21541264.2018.1523668
- Best, J. L. et. al., Identification of small-molecule antagonists that inhibit an activator:coactivator interaction, Proc Nat Ac Sci U S A, 101 (51), 17622-17627 (2004). https://doi.org/10.1073/pnas.0406374101
- Bushweller, J. H., Nat Rev Cancer, 19, 611-624 (2019). https://doi.org/10.1038/s41568-019-0196-7
- Cardenas, M. G. et. al. Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma, J Clin Invest, 126 (9), 3351-3362 (2016). https://doi.org/10.1172/jci85795
- Cerchietti, L. C. et. al. A small molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo, Cancer Cell, 17 (4), 400-411 (2010). https://doi.org/10.1016/j.ccr.2009.12.050
- Davis, I. D. et. al., Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer, N Engl J Med, 381, 121-131 (2019). https://www.nejm.org/doi/10.1056/NEJMoa1903835
- Dinkova-Kostova, A. T. et. al., Advances and challenges in therapeutic targeting of NRF2, Trends Pharmacol Sci, 44 (3), 173-149. https://doi.org/10.1016/j.tips.2022.12.003
- Filippakopoulos, P. et. al., Selective inhibition of BET bromodomains, Nature, 468 (7327), 1067-1073 (2010). https://doi.org/10.1038/nature09504
- Gerralda, E. et. al., MYC targeting by OMO-103 in solid tumors: a phase 1 trial, Nat Med, 30, 762-771 (2024). https://doi.org/10.1038/s41591-024-02805-1
- Gironda-Martínez, A. et. al., DNA-Encoded Chemical Libraries: A Comprehensive Review with Succesful Stories and Future Challenges, ACS Pharmacol Transl Sci, 4 (4), 1265-1279 (2021). https://doi.org/10.1021/acsptsci.1c00118
- Gounder, M. M. et. al., A First-in-Human Phase I Study of Milademetan, an MDM2 Inhibitor, in Patients With Advanced Liposarcoma, Solid Tumors, or Lymphomas, 41 (9), 1714-1724 (2023). https://doi.org/10.1200/jco.22.01285
- Hall, M. D. et. al. Fluorescence polarization assays in high-throughput screening and drug discovery: a review, Methods Appl Fluoresc. 4 (2), 022001 (2016). https://doi.org/10.1088/2050-6120/4/2/022001
- Jonasch, E. et. al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease, N Engl. J. Med, 385 (22), 2036-2046 (2021). DOI: doi.org/10.1056/NEJMoa2103425
- Krönke, J. et. al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells, Science, 343 (6168), 301-305 (2013). https://doi.org/10.1126/science.1244851
- Lambert, S. A. et. al., The Human Transcription Factors, Cell, 172 (4), 650-665 (2018). https://doi.org/10.1016/j.cell.2018.01.029
- Lim, K. S. et. al., Machine learning on DNA-encoded library count data using an uncertainty-aware probabilistic loss function, arXiv, 2108, 12471 (2022). https://doi.org/10.48550/arXiv.2108.12471
- Robers, M. B. et. al., Target engagement and drug residence time can be observed in living cells with BRET, Nat Commun, 6, 10091 (2015). https://doi.org/10.1038/ncomms10091
- Savitski, M. M. et. al., Tracking cancer drugs in living cells by thermal profiling of the proteome, Science, 346 (6205), 1255784 (2014). https://doi.org/10.1126/science.1255784
- Su, B. G. and Henley, M. J., Drugging Fuzzy Complexes in Transcription, Front Mol Biosci, 8, 795743 (2021). https://doi.org/10.3389/fmolb.2021.795743
- Su, L. et. al., Identification of HMGA2 inhibitors by AlphaScreen-based ultra-high-throughput screening assays, Sci Rep, 10, 18850 (2020). https://doi.org/10.1038/s41598-020-75890-0
- Tao, Z. and Wu, X. Targeting transcription factors in cancer: from “undruggable” to “druggable”, Methods Mol Biol, 2594, 107-131 (2023). https://doi.org/10.1007/978-1-0716-2815-7_9
- Tremont, A. et. al., Endocrine Therapy for Early Breast Cancer: Updated Review, Ochsner J, 17, 405-411 (2017).
- Verzella, D. et. al., The NF-κB Pharmacopeia: Novel Strategies to Subdue an Intractable Target, Biomedicines, 10 (9), 2233 (2022). https://doi.org/10.3390/biomedicines10092233
- Xie, F. et. al. Identification of a Potent Inhibitor of CREB-Mediated Gene Transcription with Efficacious in Vivo Anticancer Activity, J Med Chem, 58 (12), 5075-5087 (2015). https://doi.org/10.1021/acs.jmedchem.5b00468
- Yap, T. A. et. al. Abstract CT006: First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations, Cancer Res, 83, CT006 (2023). https://doi.org/10.1158/1538-7445.AM2023-CT006
- Zhou, H, et. al., Structure-Based Discovery of SD-36 as a Potent, Selective, and Efficacious PROTAC Degrader of STAT3 Protein, J Med Chem, 2019, 62 (24), 11280-11300 (2019). https://doi.org/10.1021/acs.jmedchem.9b01530
Related Services
| Service | |
|---|---|
Small molecule drug discovery for even hard-to-drug targets – identify inhibitors, binders and modulators | |
Molecular Glue Direct | |
PPI Inhibitor Direct | |
Integral membrane proteins | |
Specificity Direct – multiplexed screening of target and anti-targets | |
Express – optimized for fast turn – around-time | |
Snap – easy, fast, and affordable |


