Choosing the Right Inhibitor Strategy: Antagonists, Agonists, Allosteric & Covalent Inhibitors in Modern Drug Discovery
Decision makers in biotech face a familiar bottleneck: how to translate a promising biological insight into a tangible lead compound—quickly, cost-effectively, and with minimal downstream risk. Whether your team plans to run an in-house screen or outsource a DNA-encoded library (DEL) campaign to a company such as Vipergen, a clear grasp of antagonists, agonists, allosteric inhibitors, and covalent inhibitors is essential. Each class answers a different strategic question about target engagement, selectivity, dosing cadence, and liability. Below is a concise, evidence-based summary—grounded in peer-reviewed literature—that can inform your next screening and platform choice.
1. Orthosteric Antagonists: Blocking the Native Signal
Definition & sub-types
Antagonists bind the orthosteric (native-ligand) site but do not activate the receptor, thereby preventing endogenous signalling. Competitive antagonists vie directly with the agonist; non-competitive and uncompetitive antagonists lower maximal response even at saturating ligand levels. Irreversible antagonists form tight—often covalent—bonds and can block function for the protein’s lifetime.
Why they matter for screens
Decision point
2. Orthosteric Agonists: Turning the Switch On (or Down)
Agonists do more than simply “activate” a receptor; they tune the magnitude—and sometimes the direction—of the response. The matrix below captures the four principal flavours you’ll encounter when planning a screen or evaluating DEL hits.
| Agonist class | Biological effect | Typical therapeutic use | What to look for in a DEL hit |
|---|---|---|---|
| Full agonist | Drives maximal signaling | Hormone replacement, rapid bronchodilation | Orthosteric binders that fully mimic the native ligand’s H-bond map |
| Partial agonist | Sub-maximal signaling even at saturation | Smoking cessation, antipsychotics—situations where a “ceiling effect” limits side-effects | Scaffolds that preserve key contacts but soften the conformational change |
| Inverse agonist | Pushes activity below basal level; reduces constitutive signaling | Orphan GPCR research, allergy H1-blockers | Ligands that stabilise the inactive state; often reveal negative efficacy in cAMP or β-arrestin readouts |
| Biased agonist | Favour one pathway (e.g., β-arrestin) while sparing another (G-protein) | Analgesia without respiratory depression, heart-failure candidates | Chemotypes that contact both orthosteric and microswitch residues to tilt signalling balance |
Key insight: Biased and inverse agonists require signalling-specific assays—standard binding alone will miss them. Build these readouts early to avoid false negatives.
3. Allosteric Inhibitors and Modulators: Remote-Control Pharmacology
Mechanism
Allosteric inhibitors bind pockets outside the active site, inducing conformational changes that dampen activity. Negative allosteric modulators (NAMs) decrease efficacy; positive allosteric modulators (PAMs) enhance it, and some ligands exhibit ago-allosteric behaviour—weak direct activation plus modulation (Schwarts 2007).
Strategic advantages
- Superior subtype selectivity—critical when orthosteric sites are highly conserved.
- Saturable modulation—diminishing risk of overdose because allosteric effects plateau.
- Compatibility with endogenous regulation—retaining physiological “tone” rather than silencing the pathway.
Foundational review: Christopoulos outlined the structural logic of GPCR allostery, underscoring how distant pockets dictate finely tuned signalling responses (Christopoulos 2014).
Decision point
If off-target liability or resistance mutations are primary concerns, allosteric inhibitors shine. Seek DEL panels enriched in larger, three-dimensional chemotypes able to reach cryptic sites.
4. Covalent Inhibitors: Making the Interaction Stick
Irreversible vs reversible
Clinical resurgence
Modern covalent kinase inhibitors (e.g., osimertinib) illustrate that precision covalency can deliver potency with manageable toxicity. Warhead engineering focuses on tempered electrophiles (acrylamides, cyanoacrylamides) and proximity-driven selectivity.
State-of-the-art overview: Singh et al. traced the renaissance of covalent drugs and laid out warhead design rules that remain central today (Singh 2011).
5. Real-World Drug Examples by Inhibitor Class
| Class | Representative drug | Primary target / mechanism | Approved indications |
|---|---|---|---|
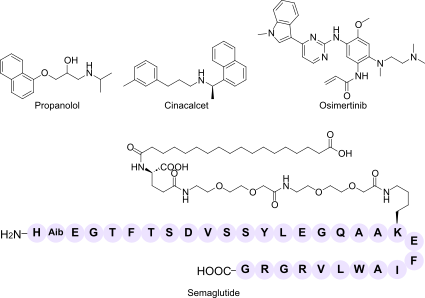
| Orthosteric antagonist | Propranolol (Inderal®) | Non-selective β-adrenergic receptor blocker that competitively antagonises epinephrine and norepinephrine | Hypertension, angina pectoris, arrhythmias, migraine prophylaxis, essential tremor |
| Orthosteric agonist | Semaglutide (Ozempic®, Wegovy®, Rybelsus®) | Full agonist of the glucagon-like peptide-1 receptor (GLP-1 R); enhances glucose-dependent insulin release and slows gastric emptying | Type 2 diabetes mellitus, chronic weight management |
| Allosteric modulator (PAM) | Cinacalcet (Sensipar®, Mimpara®) | Positive allosteric modulator of the calcium-sensing receptor (CaSR); increases receptor sensitivity to extracellular Ca²⁺ | Secondary hyperparathyroidism in dialysis patients, parathyroid carcinoma, primary hyperparathyroidism |
| Covalent irreversible inhibitor | Osimertinib (Tagrisso®) | Third-generation epidermal growth-factor receptor (EGFR) tyrosine-kinase inhibitor that forms a covalent bond with Cys797 in mutant EGFR | EGFR-mutated non-small-cell lung cancer, including T790M resistance mutation |
6. Aligning Inhibitor Class with Screening Strategy
| Strategic Question | Antagonist | Agonist | Allosteric Inhibitor | Covalent Inhibitor |
|---|---|---|---|---|
| Need for rapid proof-of-concept? | ✔ Fast orthosteric binding | |||
| Fine-tune pathway (up or down)? | ✔ Partial or Biased | ✔ NAM/PAM | ||
| High selectivity required? | ✔ Distal pocket | (✔) If unique residue is present | ||
| Desire for prolonged effect | ✔ (slow off-rate NAM) | ✔✔ |
7. Why DEL Screening is the Pragmatic Choice
DELs ley you screen hundreds of millions of compounds against your target in a single tube, capturing binders via affinity selection or Vipergen’s DEL screening in cells and decoding them by next-generation sequencing. Recent reviews documents DEL-driven discovery of nanomolar ligands across kinases, GPCRs, and protein-protein interaction inhibitors.
Vipergen’s DEL screening platform delivers:
- High Fidelity libraries – Our libraries are synthesized utilizing the Yoctoreactor which gives a complete match between DNA barcode and molecule without any truncated products.
- Highly diverse libraries, which are designed to be sp3-rich, have high 3D diversity and be rule-of-5 compliant.
- Screening inside living cells – Our proprietary technology allows us to screen directly for e.g. molecular glues, PPI inhibitors and integrated membrane proteins. See the full list of services here.
- Rapid hit-resynthesis – we can perform off-DNA resynthesis for you, for direct hit-validation.
For decision makers balancing budget, speed, and risk, a DEL campaign de-risk early discovery and delivers structure-activity clues up-front – especially when inhibitor class is still fluid.
8. Take-Home Messages
- Class drives strategy: Antagonist, agonist, allosteric inhibitor, or covalent inhibitor each solves a different pharmacological problem.
- Match assay to mechanism: Screening readouts and kinetic endpoints must align with desired mode of action.
- Leverage DEL flexibility: Modern DEL chemistry can explore every inhibitor class in parallel, collapsing timelines and cost.
- Invest in structural insight early: Orthosteric versus allosteric binding will dictate downstream medicinal chemistry.
- Partner for speed: External platforms with validated on-DNA chemistries accelerate the path to a qualified lead.
9. References
- Christopoulos, A., Advances in G Protein-Coupled Receptor Allostery: From Function to Structure, Mol. Pharmacol., 2014, 86 (5), 463-478. doi.org/10.1124/mol.114.094342
- Schwartz, T. W. et. al., Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act?, Trends Pharmacol. Sci., 2007, 28 (8), 366-373. doi.org/10.1016/j.tips.2007.06.008
- Singh, J. et. al., The resurgence of covalent drugs, Nat. Rev. Drug Discov., 2011, 10, 307-311. doi.org/10.1038/nrd3410
Related Services
| Service | |
|---|---|
Small molecule drug discovery for even hard-to-drug targets – identify inhibitors, binders and modulators | |
Molecular Glue Direct | |
PPI Inhibitor Direct | |
Integral membrane proteins | |
Specificity Direct – multiplexed screening of target and anti-targets | |
Express – optimized for fast turn – around-time | |
Snap – easy, fast, and affordable |


