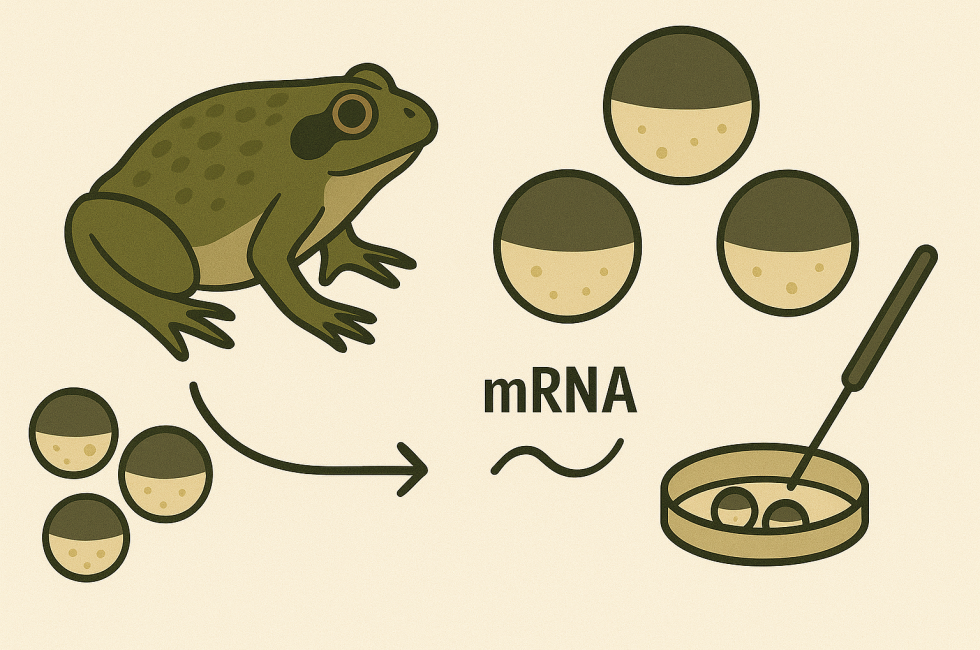
Xenopus Oocytes in Human Protein Research and Drug Discovery
Historical Background and Core Advantages
The utilization of frog oocytes, particularly those from the African clawed frog (Xenopus laevis), has become important in the study of human proteins. These oocytes, or immature egg cells, provide a robust and versatile system for the expression and functional analysis of various proteins, including ion channels, transporters, and receptors (Ivorra et al., 2022). Due to their unique biological properties and ease of manipulation, Xenopus oocytes have become an invaluable tool in biomedical research.
The scientific use of Xenopus oocytes began in the mid-20th century and was revolutionized in the 1970s with the development of mRNA microinjection techniques. Since then, these cells have been employed as a key system for in vitro expression and analysis of proteins from diverse species.
Why Xenopus oocytes?
– Natural protein synthesis machinery: Supports complex folding and post-translational modifications similar to human cells
– Wide expression capabilities: Can express challenging human proteins that do not fold or function properly in other systems
– Year-round availability: Commercial suppliers provide high-quality oocytes consistently
– Large size (1.0–1.3 mm): Easy to handle and manipulate
These features make them highly effective for studying human membrane proteins, intracellular signaling pathways, and receptor-ligand interactions.
Functional Expression and Assays
After mRNA injection, functional assays allow detailed investigation of expressed proteins. Some key methods include:
– Patch-clamp electrophysiology: Measures ion channel activity with high precision
– Radiolabeled uptake assays: Quantifies transporter function
– Ligand binding and pharmacology assays: Evaluates receptor interactions with small molecules
– DEL screening: Screening of DNA Encoded Libraries (DELs) in living cells
Applications in Human Protein and Disease Research
Xenopus oocytes are widely used to model human diseases and understand the function of human proteins, especially in the nervous system, cardiovascular system, and metabolism.
Neurological Targets
Xenopus oocytes have played a central role in characterizing key targets involved in central nervous system (CNS) disorders. Functional expression of human serotonin (5-HT) receptors, GABA_A and glutamate receptors, as well as voltage-gated potassium (Kv) and sodium (Na_v) channels, has allowed researchers to elucidate biophysical properties and pharmacological profiles critical to neuropsychiatric and neurodegenerative drug discovery. This model enables precise dose-response assessments, agonist/antagonist screening, and evaluation of modulatory compounds under near-physiological conditions. For example, selective serotonin receptor modulators, developed for mood and anxiety disorders, have been validated in oocyte assays due to their ability to maintain native receptor conformations and G-protein coupling properties (O’Connor et al., 2023).
Cystic Fibrosis and Ion Channelopathies
The functional analysis of mutated CFTR (cystic fibrosis transmembrane conductance regulator) variants in Xenopus oocytes has significantly advanced the understanding of genotype-phenotype correlations in cystic fibrosis. The system allows for rapid assessment of CFTR activity via chloride conductance assays, providing a robust platform to evaluate therapeutic candidates, including small-molecule potentiators and correctors. Oocytes expressing mutant CFTR can be treated with test compounds to validate functional rescue, a step critical in preclinical screening workflows (Kvist et al., 2011).
Diabetes and Metabolic Disorders
By co-expressing human insulin receptors (IR) with glucose transporters (such as GLUT4), Xenopus oocytes have provided an experimental model to reconstruct insulin signaling cascades. This approach has been used to study insulin-stimulated hexose uptake and to validate the effect of insulin-mimetic compounds and insulin pathway modulators. Such assays have been used to evaluate the pharmacological activity of candidate compounds targeting Type 2 diabetes and insulin resistance (Vera & Rosen, 1990).
Cardiovascular Research
Xenopus oocytes have been used to study the electrophysiology and pharmacology of cardiac ion channels such as hERG (human Ether-à-go-go-Related Gene) potassium channels, which are critical for cardiac repolarization. Functional expression of hERG in oocytes supports safety pharmacology screening by identifying compounds that may cause QT prolongation, a known risk factor for drug-induced arrhythmias. (Dascal, 1987 Yang et al., 2025).
Oncology and Cell Signaling
The oocyte model enables detailed dissection of mitogenic and apoptotic pathways by expressing components of the MAPK, PI3K/AKT, and other signal transduction pathways. Researchers have used Xenopus oocytes to analyze kinase activity, post-translational modifications, and drug-induced pathway modulation. This model supports the functional validation of small molecule inhibitors targeting oncogenic kinases and phosphatases and aids in the identification of lead compounds for cancer therapy (Dupré et al., 2011).
DNA Encoded Library (DEL) Screening in Living Cells
An innovative leap in drug discovery is the use of Xenopus oocytes for DEL screening in live cells, pioneered by Vipergen in 2021. This approach allows for high-throughput screening against proteins in their native cellular context (Petersen et al., 2021).
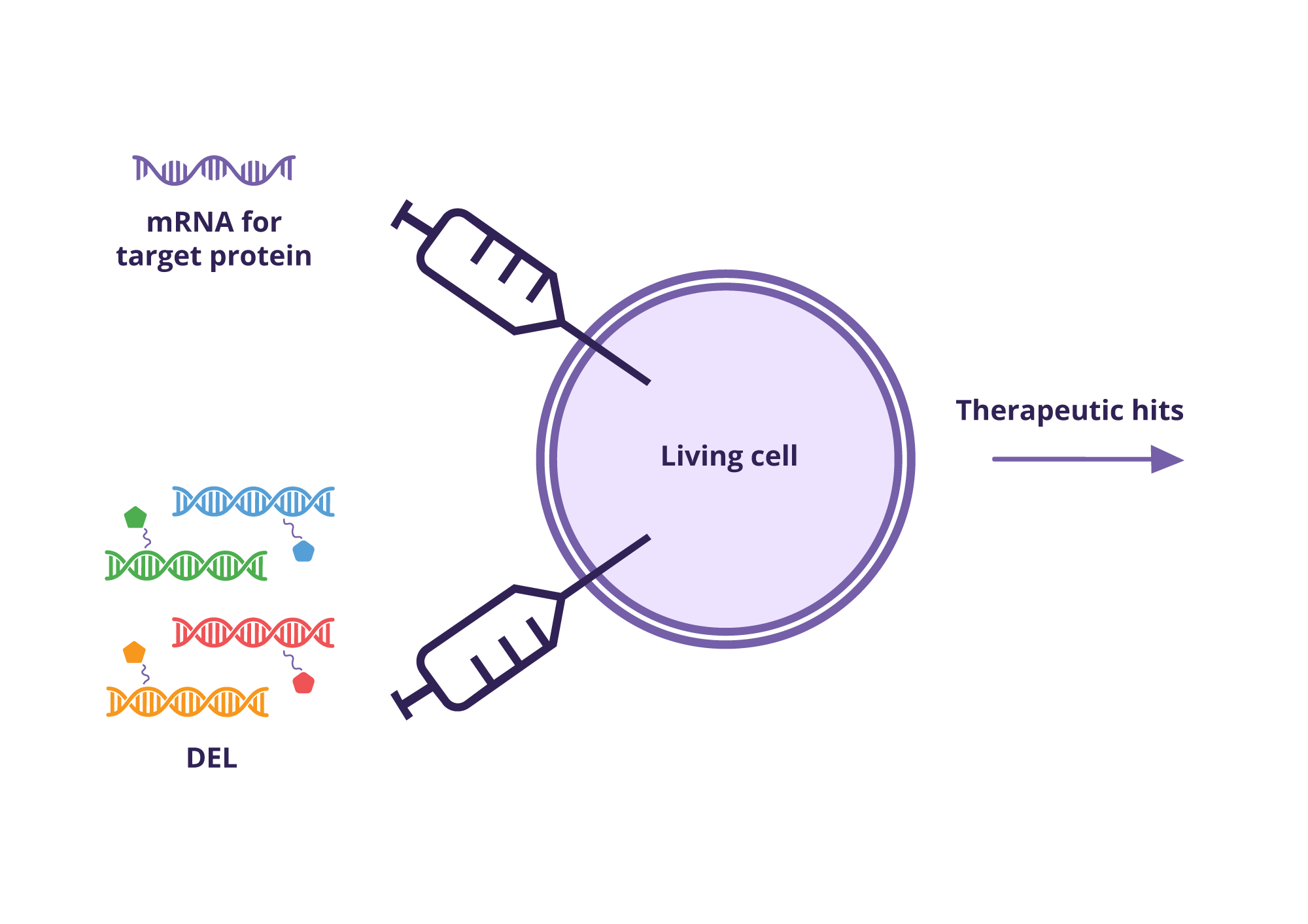
This technology is amenable for several target classes and offered by Vipergen as a DEL screening service
Conclusion
Xenopus laevis oocytes are a cornerstone of human protein research and modern drug discovery. Their utility spans basic biology, disease modeling, and compound screening including in-cell DEL screening.
References
De Robertis, E. M., & Gurdon, J. B. (2021). A Brief History of Xenopus in Biology. Cold Spring Harbor Protocols, https://doi.org/10.1101/pdb.top107615
Dascal, N. (1987). The Use of Xenopus Oocytes for the Study of Ion Channels. Critical Reviews in Biochemistry, 22(4), 317. https://doi.org/10.3109/10409238709086960
Dupré, A., Haccard, O., & Jessus, C. (2011). Mos in the Oocyte: How to Use MAPK Independently of Growth Factors and Transcription to Control Meiotic Divisions. Journal of Signal Transduction 1, Article ID 350412 https://doi.org/10.1155/2011/350412
Gurdon, J.B., Lane, C.D., et al. (1971). Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature 233, 177. https://doi.org/10.1038/233177a0
Ivorra, I, Alberola-Die, A., et al. (2022). Xenopus Oocytes as a Powerful Cellular Model to Study Foreign Fully-Processed Membrane Proteins. Membranes 12, 986. https://doi.org/10.3390/membranes12100986
Kvist, T., Hansen, K. B., & Bräuner-Osborne, H. (2011). The use of Xenopus oocytes in drug screening. Expert Opinion on Drug Discovery, 6(2), 141. https://doi.org/10.1517/17460441.2011.546396
Nutt, L. (2012). The Xenopus oocyte: A model for studying the metabolic regulation of cancer cell death. Seminars in Cell and Developmental Biology 23, 412. https://doi.org/10.1016/j.semcdb.2012.03.015
O’Connor, E. C., Kambara, K., & Bertrand, D. (2023). Advancements in the use of Xenopus oocytes for modelling neurological disease for novel drug discovery. Expert Opinion on Drug Discovery, 19(2), 173. https://doi.org/10.1080/17460441.2023.2270902
Pehl, U., Leisgen, C., Gampe, K., & Guenther, E. (2004). Automated higher-throughput compound screening on ion channel targets based on the Xenopus laevis oocyte expression system. Assay and Drug Development Technologies, 2(5), 515. https://doi.org/10.1089/adt.2004.2.515
Petersen, L. K. et. Al., 2021, Screening of DNA-Encoded Small Molecule Libraries inside a Living Cell, J. Am. Chem. Soc., 143, 7, 2751. https://doi.org/10.1021/jacs.0c09213
Vera, J. C., & Rosen, O. M. (1990). Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: Coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Molecular and Cellular Biology, 10(2), 743. https://doi.org/10.1128/mcb.10.2.743-751
Related Services
| Service | |
|---|---|
Small molecule drug discovery for even hard-to-drug targets – identify inhibitors, binders and modulators | |
Molecular Glue Direct | |
PPI Inhibitor Direct | |
Integral membrane proteins | |
Specificity Direct – multiplexed screening of target and anti-targets | |
Express – optimized for fast turn – around-time | |
Snap – easy, fast, and affordable |


