Synthesis of DNA Encoded Libraries for Drug Discovery
Introduction to DNA Encoded Libraries in Drug Discovery
Traditionally high throughput screening of large compound collections has been the starting point for many drug discovery campaigns. This method is time-consuming and can be resource intensive if robotic setups are not utilized. Furthermore, access to large compound collections is limited, and usually only large pharmaceutical companies have access to the vast number of compounds required for comprehensive screening. One solution to the challenges associated with high-throughput screening is DELs, which offer a more efficient and cost-effective approach to screening millions of compounds in parallel.
What is a DNA-Encoded Library (DEL)?
A DNA-Encoded Library (DEL) is a collection of small molecules, each covalently linked to a short DNA barcode that records its synthesis. DEL technology uses an affinity-selection workflow that integrates chemistry, biology, computational chemistry, and bioinformatics to screen pooled libraries in a single tube and access more chemical space at lower cost. Because hits are read out by next-generation sequencing (NGS), billions of barcoded compounds can be screened with very little target protein and minimal assay development—breaking the traditional “cost-per-well” model. The result is rapid, accurate hit identification for modern drug discovery.
Figure 1: Typical 3-cycle DEL compound
Early History of DNA Encoded Libraries
Following the rise of combinatorial chemistry, Brenner and Lerner conceptualized DNA encoding of small molecules in 1992 (Brenner and Lerner, 1992). Soon after, in 1993 the first chemistry was developed, in which a peptide library was constructed on glass beads carrying a DNA barcode (Nielsen, 1993). Following this no significant advancements were made until around 2004, when the first methodologies for construction of DELs without the use of beads were reported:
- David Liu’s group introduced DNA-templated synthesis (Gartner, 2004).
- Dario Neri’s group introduced encoded self-assembling chemical libraries (Melkko, 2004).
- Harpin and Harbury introduced DNA routed synthesis (Harpin and Harbury, 2004).
A few years after these initial discoveries Morgan and co-workers at Praecis Pharmaceuticals (now GSK) introduced the first Split-and-Pool derived DEL, which is today the most used technology for DEL synthesis (Clark, 2009). At the same time Vipergen introduced an alternative method for DEL synthesis using 3D DNA junctions to template DEL synthesis (Hansen, 2009).
Synthesis and Encoding Strategies for DNA Encoded Libraries
Multiple techniques exist to construct DELs, and novel approaches also appear in literature today. There are, however, a set of methods that have proven powerful and are used today to construct DELs both in academia as well as in pharmaceutical companies. The most prominent methods are described below.
Split-and-Pool Synthesis Explained
Split-and-Pool Synthesis is the most widely used technique for constructing DELs (Figure 2A). Here, the library is constructed stepwise by subsequent chemical reactions and DNA encoding. Following each encoding step, the library is pooled together and mixed before it is split into new fractions for the subsequent chemical step. If N building blocks are utilized in each round (R) of split-and-pool synthesis the library will consist of NR library members. Three rounds of synthesis with 100 building blocks in each round will result in a DEL containing 106 different molecules. If one additional round is added the library will have 108 different molecules. It might seem compelling to add more rounds of synthesis, but practically once the synthesis exceeds 3-4 rounds the molecules identified will have a high molecular weight which falls outside of the drug-like chemical space.
The major pitfall of DEL synthesis by Split-and-Pool is the risk of truncated products. As the encoding (e.g. by ligation) of DNA happens independently of the chemical reaction each individual molecule will carry the DNA-tag even if the chemical reaction has not taken place. This puts limits on which chemical reaction can take place as they need to be high yielding and not generate side products.
Encoded Self-Assembling Chemical Libraries
Encoded Self-Assembling Chemical (ESAC) Libraries rely on single stranded DNA to form duplexes when paired with a complementary strand. This allows for the construction of sub-libraries containing a constant region which has a complementary hybridization strand, which forms duplexes with the other sub-libraries. If each sub-library contains X and Y members respectively, the total number of library members will be X×Y. ESAC libraries can be utilized in many ways allowing for a flexible screening platform:
- Two sub-libraries can be assembled allowing for identification of de novo bidentate molecules. Though this might seem appealing, the initial follow-up work needs to merge these individual ligands together, which can be challenging.
- A non-coding oligonucleotide can be used to make a sub-library double stranded. This will give display of one of the sub-libraries which will resemble a classic DEL.
- Pairing a known binder with a sub-library allows for the maturation of a hit compound.
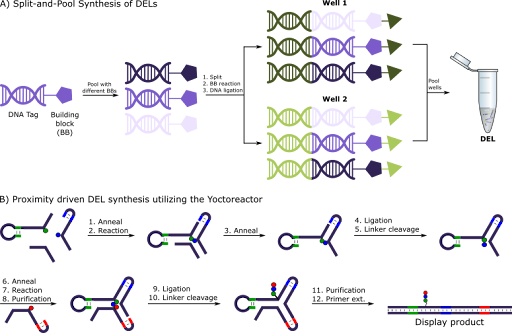
Figure 2: Synthesis of DNA Encoded Libraries by A) Split-and-Pool Synthesis or B) Proximity driven utilizing the Yoctoreactor
DEL Synthesis Utilizing the Proximity-Driven YoctoReactor® Technology
To circumvent truncations and poor reactivity of some building blocks, Vipergen has developed the YoctoReactor (yR) technology (Figure 2B, Hansen 2009). The yR exploits the inherent capabilities of DNA to self-assemble into complex 3D structures. Bringing chemical building blocks into proximity, directed by DNA, allows for highly efficient synthesis of DNA encoded libraries. Chemical building blocks are attached to a DNA region, which besides encoding for the building block also contains a non-coding region which directs the yR self-assembly when mixed with complimentary building blocks allows for the self-assembly of the yR. The building blocks can be attached to the DNA via either a cleavable or non-cleavable linker specified by the attachment point to the DNA.
Initially two building block-DNA conjugates self-assembles with a helper oligo to form the yR. The two chemical building blocks are brought into proximity in the DNA junction, where they can react. Following chemical reaction, the conjugates are purified, and the DNA is ligated before the linker of one building block is cleaved. Next, the third building block can be added and reacted. Again, the conjugates are purified, before the DNA is ligated and the linker is cleaved. Finally, purification followed by primer extension yields the final display product.
By introducing a purification step after the two building blocks have reacted, but before the DNA is ligated, this allows for unreacted building blocks to be removed from the library. This ensures that any truncated products will not be part of the final library, meaning that there will be a complete match between the DNA code and the displayed product.
How does Vipergen’s DEL technology for DEL synthesis differ from classical methods?
Utilizing a fully templated synthesis like the Yoctoreactor® DEL technology fully eliminates truncates arising from poor reactivity of some building blocks. This gives a full correlation between compound and barcode, which allows us to perform more complex screens without worrying about false positives. In a publication Vipergen researchers identified inhibitors from a yR-library (Lib022 containing 12.6 million different compounds). The compounds selected for off-DNA synthesis had a low false-positive rate and 22 of 24 showed some inhibitory potency in enzymatic assays (Petersen 2016).
DNA routing in DEL technology
Where Split-and-Pool synthesis is described as DNA-recorded, utilizing the YoctoReactor can be classified as DNA-templated DEL synthesis as the DNA sequence and formation of the 3D template drive library construction. Other methods of DNA-templated DEL synthesis exist, e.g. by Halpin and Harbury, who developed hybridization columns which can capture DNA onto Sepharose resin on which the chemical reaction can be performed (Halpin and Harbury 2004). A similar technique was developed by Li and co-workers, who introduced a universal DNA template, which can hybridize with non-specific reagent DNAs containing different sequences (Li 2013). In this way, one DNA string can be used to template the entire synthesis.
Applications of DELs in Modern Drug Discovery
DNA-Encoded Libraries (DELs) have transformed the landscape of early-stage drug discovery by enabling the rapid identification of small-molecule binders across a broad range of biological targets. Beyond their utility as a screening platform, DELs have been integrated into various stages of hit identification and optimization, with growing relevance in both academic and industrial settings.
One of the key advantages of DEL technology lies in its capacity to screen billions of compounds simultaneously using minimal amounts of target protein. This has made DELs especially attractive for targets that are challenging to access using traditional high-throughput screening (HTS) methods. These include:
- Protein–protein interactions (PPIs): DELs have been successfully applied to identify small molecules that modulate or inhibit PPIs, a target class traditionally considered undruggable.
- Difficult or low-abundance targets: DELs can be used even when target protein is available only in small quantities, such as membrane proteins, nuclear receptors, and multi-subunit complexes.
- Fragment-based approaches: DELs can be used to explore vast chemical space with small, fragment-like building blocks, which can be further optimized post-screening.
Advantages of DNA-encoded libraries in hit identification
DELs enable ultra-scalable, cost-efficient hit identification. Hundreds of millions of small molecules – each barcoded with a unique DNA code – are screened together by affinity selection, greatly increasing the chance of finding high-quality binders. Hits are decoded by NGS, delivering rapid readouts, rank-ordering by enrichment, and immediate SAR hypotheses. DELs require only tiny amounts of target protein and minimal assay development, reducing “cost-per-well” to near zero compared to traditional High Throughput Screening. Because binding is measured directly in solution, DEL screening suits challenging targets and native-like conditions. Libraries spanning fragments to macrocycles expand the chemical space and streamline off-DNA resynthesis from the recorded synthetic route.
Vipergen’s technologies completely omits the need for purified protein, by in cell screening. Read more about DEL Screening here and Vipergen’s DEL screening services.
Advancing from Hits to Leads
Once potential binders are identified through DEL selection and decoding, off-DNA synthesis of hits enables their validation in orthogonal biochemical or biophysical assays. Many companies have incorporated DEL-derived hits into their lead optimization workflows, where these compounds undergo structure–activity relationship (SAR) exploration and pharmacological profiling.
DELs have also played a role in:
- Allosteric modulator discovery, where hits bind to non-canonical sites (Ahn 2017)
- Covalent inhibitor development, through libraries containing reactive electrophiles (Dickson 2024, Huang 2025)
- Structure-based drug design, especially when DEL hits are co-crystallized with targets (Huang 2025)
Target Class Versatility
DELs have been successfully used across various target classes, including:
- Kinases
- G-protein-coupled receptors (GPCRs)
- Integral membrane proteins
- Proteases
- Epigenetic regulators
- RNA-binding proteins
- Transcription factors
This versatility makes DELs a powerful complementary approach to traditional screening platforms, especially when combined with structural biology or AI-driven hit expansion.
The market for DNA-Encoded Libraries and future perspective
The DEL market has moved from niche to mainstream as pharma- and biotech companies seek faster and cheaper hit discovery. Recent analysis places the market around $0.75B in 2024 and an expected to grow substantially in the near future (Grand View Research 2025). Looking ahead, three trends stand out. First, data-centric DEL: machine learning on selection readouts is improving triage, SAR extraction, and hit enrichment, with prospective studies validating DEL+ML pipelines (Iqbal 2025). Second, chemistry expansion: continued growth of DNA-compatible transformations, macrocycle/ring-closing strategies, and on-/off-DNA synthesis hybrids will widen accessible chemical space and drug-likeness (Ma 2024). Third, new selection contexts: movement toward cellular and native-like systems promises better target engagement fidelity and applicability to previously intractable targets (Peterson 2023).
Taken together, DEL is set to remain a core front end for discovery, complementing HTS, fragment platforms, and structure-based design. As datasets scale and workflows integrate AI and automated DMTA loops, the competitive edge will hinge on library quality, informatics, and translational selection models rather than sheer library size (Peterson 2023).
Conclusion
DNA-Encoded Libraries (DELs) represent a transformative advancement in early-stage drug discovery, offering an efficient and scalable means to explore vast chemical space. Through diverse synthesis strategies such as split-and-pool and proximity-driven approaches like Vipergen’s YoctoReactor, DELs enable the rapid identification of small-molecule binders against a wide range of biological targets. Each method offers unique advantages—split-and-pool synthesis provides unparalleled diversity, while proximity-driven methods offer higher fidelity and reduced truncation artifacts. Together, these complementary technologies allow researchers to tailor DEL campaigns to specific targets, chemical constraints, or discovery goals. As DEL platforms continue to evolve, they are expected to play an increasingly central role in hit discovery, target validation, and the acceleration of lead optimization efforts.
FAQ
DNA-Encoded Libraries (DELs) are used for ultra-high-throughput hit discovery in drug research. Billions of small molecules, each tagged with a DNA barcode, are screened en masse against proteins, nucleic acids, or cells by affinity selection. Bound molecules are identified by next-generation sequencing of their barcodes, enabling rapid, cost-efficient prioritization of chemotypes and early structure–activity relationships. DEL selections support target validation, fragment-to-lead campaigns, macrocycle discovery, and exploration of hard-to-drug sites with minimal protein consumption and minimal assay development.
A preferred DNA-encoded library (DEL) size is roughly 500 million to 1 billion members for drug discovery, balancing search breadth with sequencing/data quality. Bigger isn’t automatically better: performance depends on chemical diversity (scaffolds, functional groups, stereochemistry), drug-like properties (molecular weight, clogP, HBD/HBA), and encoding fidelity. Coverage of 3D shape and Fsp³, tractable exit vectors, and ease of off-DNA resynthesis also matter. Finally, fit to the target class and selection format (solution or cell-based) is critical—smart design beats sheer size.
DELs are typically built by iterative split–pool synthesis on DNA: a scaffold or linker is attached to DNA, subpools react with diverse building blocks using DNA-compatible chemistries, and each step is recorded by ligating or polymerase-extending a unique DNA tag. Alternative methods includes the Yoctoreactor technology which templates the DEL synthesis hereby eliminating truncates.
DNA-Encoded Libraries (DELs) accelerate drug discovery by compressing screening and hit identification into a single pooled affinity-selection experiment. Billions of small molecules, each tagged with a DNA barcode, are tested at once; binders are read out by next-generation sequencing (NGS), turning weeks of plate-based assays into days. DELs require tiny amounts of target protein and minimal assay development, slashing “cost-per-well” and enabling rapid prioritization of chemotypes and early SAR. The result is faster, lower-risk progression from target to validated leads.
The Yoctoreactor® allows for the templated synthesis of DNA encoded libraries inside self-assembled 3D-junctions. Critically, the Yoctoreactor delivers libraries with high fidelity – one-to-one correspondence between barcode and small molecule – which reduces false positives and simplifies off-DNA resynthesis. Paired with Vipergens (cellular) binder trap enrichment it supports rapid selection utilizing either tiny amounts protein or directly inside living cells eliminating the need for purified protein altogether.
Read moreReferences
- Ahn, S., et. al. (2017), Allosteric “beta-blocker” isolated from a DNA-encoded small molecule library, Proc. Natl. Acad. Sci. USA, 114 (7), 1708-1713. doi.org/10.1073/pnas.1620645114
- Brenner, S. and Lerner, R. A. (1992), Encoded combinatorial chemistry. Proc. Natl. Acad. Sci. USA, 89, 5381-5383. doi.org/10.1073/pnas.89.12.5381
- Dickson, P. (2024), DNA-Encoded Library Technology – A Catalyst for Covalent Ligand Discovery, ACS Chem. Biol, 19, 4, 802-808. doi.org/10.1021/acschembio.3c00803
- Gartner, Z. J. et. al. (2004), DNA-Templated Organic Synthesis and Selection of a Library of Macrocycles. Science, 305, 1601-1605. doi.org/10.1126/science.1102629
- Halpin, D. R. and Harbury, P. B. (2004), DNA Display I. Sequence-Encoded Routing of DNA Populations, PLoS Biology, 2 (7), 1015-1021. doi.org/10.1371/journal.pbio.0020173
- Halpin, D. R. and Harbury, P. B. (2004), DNA Display II. Genetic Manipulation of Combinatorial Chemistry Libraries for Small-Molecule Evolution, PLoS Biology, 2 (7), 1022-1030. doi.org/10.1371/journal.pbio.0020174
- Hansen, M. H. et. al. (2009), A Yoctoliter-Scale DNA Reactor for Small-Molecule Evolution, J. Am. Chem. Soc., 131 (3), 1322-1327. doi.org/10.1021/ja808558a
- Huang, D., et. al. (2025), Identification of Structurally Novel KRASG12C Inhibitors through Covalent DNA-Encoded Library Screening, J. Med. Chem., 68 (4), 4801-4817. doi.org/10.1021/acs.jmedchem.4c03071
- Iqbal, S., et. al. (2025), Evaluation of DNA encoded library and machine learning model combinations for hit discovery, npj Drug Discov. 2, 5. doi.org/10.1038/s44386-025-00007-4
- Li, Y., et. al. (2013), Multistep DNA-Templated Synthesis Using a Universal Template. J. Am. Chem. Soc., 135, 17727-17730. doi.org/10.1021/ja409936r
- Ma, P., et. al. (2024), Evolution of chemistry and selection technology for DNA-encoded library, 14 (2), 492-516. doi.org/10.1016/j.apsb.2023.10.001
- Melkko, S. et. al. (2004), Encoded Self Assembling Chemical Libraries. Nat. Biotechnol., 22, 568-574. doi.org/10.1038/nbt961
- Nielsen, J., Brenner, S., Janda, K. D. (1993), Synthetic methods for the implementation of encoded combinatorial chemistry. J. Am. Chem. Soc., 115, 9812-9813. doi.org /10.1021/ja00074a063
- Petersen, L. K. et. al. (2016), Novel p38α MAP kinase inhibitors identified from yoctoReactor DNA-encoded small molecule library, Med. Chem. Commun., 7, 1332-1339. doi.org/10.1039/C6MD00241B
- Peterson, A. A. and Lui, D. (2023), Small-molecule discovery through DNA-encoded libraries, Nat. Rev. Drug Discov. 22, 699-722. doi.org/10.1038/s41573-023-00713-6
Related Services
| Service | |
|---|---|
Small molecule drug discovery for even hard-to-drug targets – identify inhibitors, binders and modulators | |
Molecular Glue Direct | |
PPI Inhibitor Direct | |
Integral membrane proteins | |
Specificity Direct – multiplexed screening of target and anti-targets | |
Express – optimized for fast turn – around-time | |
Snap – easy, fast, and affordable |


